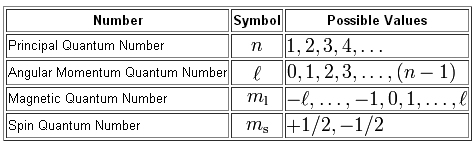
There are four different types of quantum numbers used to decsribe an electron present in an orbital. The four quantum numbers are a Principal quantum number n b Azimuthal quantum number l c Magnetic quantum number m d Spin quantum number s.

Quantum Number Orbital Definition Formula Diagram Shape
The allowed values of n are therefore 1 2 3 4 and so on.

. They are Principal quantum numberAzimuthual quantum numberMagnetic quantum number and Spin quantum number. Explain quantum numbers and the atomic structure. If n 3 for example l can be either 0 1 or 2.
Azimuthal or Angular momentum Quantum Number. An important family is flavour quantum numbers internal quantum numbers which determine the type of a particle and its interactions with other particles through the fundamental forces. These are of four typesPrincipal Quantum number nAzimuthal Quantum Number iotaMagnetic Quantum Number m_iotaSpin Quantum Number ms Each of these quantum numbers describes a specific feature of electron.
Describe the Pauli exclusion principle. Each of these quantum numbers cover a quantum mechanical principle and together forms the basis of the branch. 1 1 12.
Different values of l correspond to different types of subshells. Azimuthal Quantum number- represented by l. Azimuthal Quantum Number l.
The Quantum numbers are a set of protocols that are used to describe the position and energy of an electron in an atom. Magnetic Quantum Number m. There are four quantum numbers.
Electrons present in an atom are described by a set of four numbers which are collectively known as quantum numbers. Magnetic Quantum number - represented by m. Explain different types of diatomic bonds.
1 1 12. There are four quantum numbers. Thus this number helps to explain the fine lines of the spectrum.
The principal quantum number n cannot be zero. There are four of these numbers the principal quantum number the angular momentum quantum number also known as the orbital quantum number the magnetic quantum number and the electron spin quantum number. The azimuthal quantum number l is related to the geometrical shape of the orbital.
For example the 3d subshell is in the n 3 shell the 2s. You will understand how to find the principal quantum number n the angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms. There are four quantum numbers necessary to describe an electron Principal quantum number n designates size Angular moment quantum number l-describes shape Magnetic quantum numbers ml - specifies orientation Spin Quantum Number ms describes the spin of the.
It can have ant positive value 123 and so on. Within the same principal Shell there are present a number of sub shells or sub levels of energy. M s or s - spin quantum number.
How to Find the Quantum Numbers of an Element. The four quantum numbers are the principle quantum number n the angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms. As a result the number of electronic jumps increases and so is the number of lines.
Explain the structure and properties of solids. N 4 number of electrons. Azimuthal quantum number tells about the.
The allowed values and general meaning of each of the four quantum number of an electron numbers of an electron in an atom are as follows. Quantum numbers often describe specifically the energy levels of electrons in atoms but other possibilities include angular momentum spin etc. These are a group of numerical values which provide solutions that are acceptable by Schrodinger wave equation for hydrogen atoms.
Quantum Numbers and types 1 Principal quantum numbers 2 Azimuthal quantum numbers 3 Magnetic quantum numbers 4 Spin quantum numbers. This quantum number gives the orbital in which an electron is present and also the number of. N - principal quantum number.
Solve any question of Atoms with-. Quantum numbers are also used to determine other characteristics of atoms such as ionization energy and the atomic radius. M ℓ or m - magnetic quantum number.
Any quantum system can have one or. The angular quantum number l can be any integer between 0 and n - 1. 1Principal quantum number nThis quantum number is the one on which the energy of an electron in an atom principally depends.
The energy of an electron in an atom depends on. The four quantum numbers are the principle quantum number n the angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms. To completely describe an electron in an atom four quantum numbers are needed.
This quantum number gives information for the subshell in which electron is present the number of subshellsthe shape of the subshell and also the orbital angular momentum of the electron. Describe the Pauli exclusion principle. Describes the energy level.
Explain different types of diatomic bonds. The numbers which assign the position and energy to an electron are called quantum numbers. The magnetic quantum number m can be any integer between.
The first quantum number describes the electron shell or energy level of an atom. Spin Quantum Number s. Quantum Number n.
Ill explain the four different types of quantum numbers you will need to know. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s. The principle quantum number n describes the energy and distance from the nucleus and represents the shell.
Atomic orbitals can be completely described in terms of a set of three quantum numbers n l m s. Explain quantum numbers and the atomic structure. Quantum numbers are required to describe the distribution of electron density in an atom.
ℓ - azimuthal or angular momentum quantum number. The value of l may be zero or a positive integer less than or equal to n1 n is the principal quantum number. Principal quantum number gives us information about the size and energy of the orbital.
There is a set of four quantum numbers associated with each electron of an atom. There are four quantum numbers the principal quantum number n azimuthal quantum number l the magnetic quantum number m and spin quantum number s. Quantum numbers are important because they can be used to determine the electron configuration of an atom and the probable location of the atoms electrons.
Describes the orbital of the subshell.
Quantum Numbers Introduction To Chemistry

How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube

0 Comments